In-depth Exploration of m-Xylene: Properties and Impacts

Intro
m-Xylene, one of the three isomers of xylene, plays a significant role in diverse industrial applications. As an aromatic hydrocarbon, it possesses unique chemical properties that make it vital for various sectors, including chemicals, plastic, and petrochemicals. Understanding m-xylene is not just important for chemists but also for those involved in industrial design and environmental science. This article aims to dissect the properties, applications, and impacts of m-xylene comprehensively.
Methodologies
Description of Research Techniques
In examining m-xylene, several research methodologies are employed. These techniques range from physical and chemical analysis to more advanced spectroscopic methods. Chromatography, for instance, helps in identifying and quantifying m-xylene in various samples. Nuclear Magnetic Resonance (NMR) spectroscopy gives insights into the molecular structure, while Gas Chromatography-Mass Spectrometry (GC-MS) is crucial for analyzing complex mixtures containing m-xylene.
Tools and Technologies Used
The study of m-xylene involves specific tools and technologies. High-performance liquid chromatography (HPLC) is frequently used to separate and analyze compounds, ensuring accurate identification. In addition, advanced modeling software may simulate chemical behavior under different conditions, facilitating better understanding of its properties. This combination of methodologies and tools provides a robust framework for analyzing m-xylene in a scientific context.
"Understanding the methodologies applied to study m-xylene equips researchers and industry professionals with the knowledge to manipulate this compound effectively."
Discussion
Comparison with Previous Research
Previous studies on m-xylene often focused on its synthesis and economic viability. However, recent research has addressed its environmental and health impacts in more detail. Comparing these findings helps to contextualize current knowledge and highlight gaps in understanding, especially regarding emissions and exposure limits.
Theoretical Implications
Theoretical implications of m-xylene research are significant. As an essential compound in organic synthesis, insights gained can affect various chemical processes, leading to innovations in polymers and solvents. Additionally, research can inform safer handling practices and regulatory policies that govern m-xylene use.
Understanding m-xylene involves navigating its complex chemical properties and examining how they translate into real-world applications and consequences for health and the environment. Not only does this enhance comprehension of the compound itself, but it also underscores its importance in industrial chemistry and environmental science.
Prelude to m-Xylene
m-Xylene is an important aromatic hydrocarbon with significant applications across various industries. Understanding its characteristics and utilities is essential for students, researchers, educators, and industry professionals who are involved in chemistry or environmental studies. This section introduces m-Xylene by delving into its definition and chemical structure, as well as its historical context, providing a backdrop that enhances the reader's grasp of its relevance in contemporary settings.
Definition and Chemical Structure
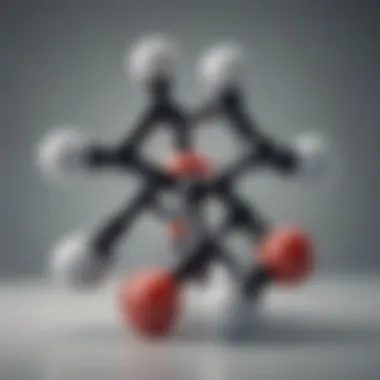
m-Xylene, also known as 1,3-dimethylbenzene, is one of the three isomers of xylene. Its chemical formula is C80, indicating that it contains two methyl groups attached to a benzene ring. The arrangement can be depicted as:
This structural configuration contributes to its properties and reactivity. m-Xylene is typically a colorless, flammable liquid with a sweet odor. It has a lower density than water, making it insoluble in water but soluble in organic solvents. This compound's physical properties play a crucial role in its extensive applications, ranging from solvents and chemical production to its use in fuel formulations.
Historical Context
The significance of m-Xylene can be traced back to the early 20th century when it began being utilized in various industrial processes. Discovered in the late 19th century, the compound's industrial use increased following advancements in organic chemistry, particularly in the fields of petrochemicals and plastics. Initially, the production of m-Xylene was largely linked to the distillation of coal tar; however, the advent of modern refining methods allowed for more efficient extraction from crude oil.
The evolution of m-Xylene's applications reflects broader trends in industrial growth. Its role as a solvent and intermediates in chemical synthesis is critical and has expanded with innovation in materials science. The compound is now integral to the production of phthalic anhydride, which is essential for manufacturing plastics and resins. Understanding this historical context is vital as it shapes the current industrial landscape and the ongoing research focused on improving sustainable practices related to m-Xylene.
"m-Xylene is not just a chemical; it is a cornerstone of modern industrial chemistry and environmental studies."
Chemical Properties of m-Xylene
Understanding the chemical properties of m-xylene is essential for anyone working in chemistry or related fields. The characteristics of m-xylene play a significant role in its applications and its behavior in various environments. This section examines physical and chemical properties, both of which are critical in evaluating m-xylene’s usefulness and safety in industrial applications.
Physical Properties
Melting Point
The melting point of m-xylene is approximately -47.87 °C. This low melting point indicates that m-xylene remains in a liquid state at ambient temperatures, making it suitable for various applications. The key characteristic of the melting point is its influence on the handling and storage of m-xylene. For instance, its low melting point allows m-xylene to be used in low-temperature operations, which can be beneficial in chemical processing. However, this feature also means that strict temperature control is necessary to prevent solidification during storage.
Boiling Point
The boiling point of m-xylene is about 139.12 °C. This property is significant because it dictates the temperature range in which m-xylene can operate effectively. The high boiling point makes it a good solvent for many chemical reactions and processes, as it does not evaporate quickly under standard conditions. This stability under heat is advantageous in many industrial applications including the production of paints and coatings. However, volatility can become a concern when m-xylene is exposed to high temperatures, leading to evaporation and potentially hazardous conditions.


Density
The density of m-xylene is roughly 0.865 g/cm³. Density is crucial as it affects the behavior of m-xylene when mixed with other substances. This property allows m-xylene to function effectively in various mixtures and ensures its efficiency as a solvent. Furthermore, the density helps in transportation and formulation processes, as it can influence the design of containers and determining shipping costs. It's essential to consider density in relation to environmental safety, especially when m-xylene is released into waterways.
Chemical Reactivity
Isomerization
Isomerization of m-xylene involves the rearrangement of its molecular structure without changing its molecular formula. This property is relevant because it can lead to the production of different isomers that may be more useful for specific applications. For example, the ability to convert m-xylene into its other isomers can enhance its utility in producing various chemical products. However, the process must be controlled, as excessive isomerization can lead to byproducts that may be less desirable.
Oxidation
Oxidation of m-xylene occurs when the compound reacts with oxygen, resulting in various products depending on conditions and catalysts used. This reaction is significant in the production of chemicals for perfumes and flavorings. The ability of m-xylene to undergo oxidation facilitates its role in synthesizing horticultural products. However, safety precautions are essential, as the reaction can produce toxic byproducts, requiring careful monitoring and control measures in industrial settings.
Electrophilic Substitution
Electrophilic substitution is a fundamental reaction for m-xylene, where an electrophile replaces a hydrogen atom in the aromatic ring. This characteristic contributes to the synthesis of many valuable compounds, including plastics and dyes. Electrophilic substitution allows for diverse applications, helping to extend the usefulness of m-xylene in the chemical industry. However, it requires precise conditions to ensure selectivity and yield of the desired products.
Synthesis of m-Xylene
The synthesis of m-xylene plays a crucial role in understanding its wider applications and relevance in various industries. This section will cover significant methods to produce m-xylene, focusing on both industrial and laboratory techniques. Each method offers unique benefits and considerations, impacting the efficiency, cost, and environmental ramifications of its production.
Industrial Methods
Alkylation of Toluene
Alkylation of toluene is a prominent method for synthesizing m-xylene. It involves introducing additional methyl groups to toluene, typically using a solid acid catalyst like zeolites. This process significantly contributes to the overall availability of m-xylene in the market. One key characteristic of this process is its efficiency in producing high yields of m-xylene while minimizing by-products.
The process is beneficial because it utilizes toluene, which is readily available from petroleum refining. The direct integration of toluene into the production stream helps maintain lower costs for m-xylene synthesis.
However, the alkylation process has environmental considerations. The use of acids in the reaction can lead to waste management issues if not handled properly. Thus, while effective, care must be taken to ensure that regulations surrounding such processes are adhered to.
Catalytic Reforming
Catalytic reforming is another industrial method used to produce m-xylene and is particularly significant in oil refineries. This process transforms naphtha into high-octane reformate through the catalytic activity of metal catalysts. A key feature of catalytic reforming is its ability to produce not only m-xylene but also other valuable aromatic hydrocarbons, making it a versatile option in industrial settings.
The benefit of this method lies in its ability to increase the octane rating of fuels while simultaneously generating valuable chemical feedstocks. Given its dual-purpose role, it is considered a popular choice among manufacturers seeking efficiency.
Nevertheless, catalytic reforming does have disadvantages. The process requires high temperatures and pressures, which can increase operational costs and pose safety risks. Proper management of these factors is essential to maximize output safely and sustainably.
Laboratory Synthesis
Laboratory synthesis of m-xylene provides insights into alternative methods that may be useful for small-scale production or specialized applications. This section will discuss two primary laboratory synthesis techniques: synthesis from benzene and alternative routes.
Synthesis from Benzene
Synthesis from benzene involves methylating benzene to produce m-xylene. This reaction typically requires the use of a catalyst such as aluminum chloride. This method is notable for its straightforward approach to creating m-xylene in a controlled laboratory environment. The simplicity of the reaction is what makes it an attractive option for researchers and educators who need to demonstrate foundational concepts of organic chemistry.
While the method is effective in producing m-xylene, it can also lead to undesired products if reaction conditions are not monitored closely. Careful control over temperature and catalyst concentration is essential for maximizing yield and purity of m-xylene from this process.
Alternative Routes
Alternative routes to synthesizing m-xylene include various less common methods. These may involve different starting materials or catalysts that are not traditionally used in industrial settings. The significance of exploring alternative routes lies in potential innovation and the discovery of more sustainable practices.
The characteristic feature of these methods is their experimental nature, which allows for flexibility in chemical exploration. They can offer valuable insights into more sustainable or cost-effective processes. However, these routes often lack the commercial viability and scalability of established methods. This means while they can be informative and beneficial for research, they may not replace conventional synthesis methods in an industrial context.
Exploring diverse synthesis methods not only enhances the understanding of m-xylene but also fosters innovation in the industry as researchers look for ways to improve efficiency and sustainability.
Applications of m-Xylene
The applications of m-xylene are crucial for understanding its role in various industries. This compound is more than just a chemical; it serves specific functions across physics and chemical domains. Its solvency and chemical properties make it a preferred choice in many formulations. These applications range from solvent uses in paints and coatings to its role in chemical production and fuel formulations. Each application highlights the versatility of m-xylene and its impact on industrial processes.
As a Solvent


Paints and Coatings
In the realm of paints and coatings, m-xylene offers excellent solvency properties. It is commonly used to dissolve various resins and pigments, which leads to enhanced performance of the final product. The low volatility of m-xylene also contributes to its effectiveness as a solvent, resulting in smoother finishes and improved application processes. Its ability to blend with oil-based paints sets it apart, allowing for better flow and leveling on surfaces. This unique feature is beneficial in achieving a high-quality appearance.
However, there are some disadvantages. The use of m-xylene in large amounts can lead to health risks for workers due to inhalation, requiring adequate safety measures. Despite this, its benefits in paints and coatings cannot be overstated.
Adhesives
Adhesives also rely significantly on m-xylene for their effectiveness. Its solvency allows for the proper mixing of polymer substances, ensuring strong bonding characteristics. Manufacturers favor m-xylene for creating strong and durable adhesives that can withstand various environmental conditions. This characteristic is crucial for achieving longevity and reliability in products that utilize these adhesives.
A unique feature of m-xylene in adhesives is its ability to enhance the adhesion capabilities when mixed with other solvents. Though it has an pungent odor, which might deter some users, its advantages in producing strong bonds usually outweigh such concerns.
Production of Chemicals
Production of Phthalic Anhydride
Phthalic anhydride production is another vital application of m-xylene. This chemical is primarily derived from the oxidation of m-xylene and is an important building block in the manufacture of plasticizers, resins, and dyes. The ability to produce phthalic anhydride efficiently from m-xylene highlights its role in industrial chemistry.
The key characteristic of phthalic anhydride production is its high yield when utilizing m-xylene. This feature makes it an economically favorable choice for large-scale operations, reducing waste and enhancing productivity. However, one must consider the environmental implications of its production, as the oxidation processes can lead to emissions that require monitoring.
Synthesis of Xylene Derivatives
Synthesis of xylene derivatives also utilizes m-xylene as a starting material. The derivatives produced from m-xylene can be employed in a variety of industrial applications, from plastics to pharmaceuticals. This shows the versatility of m-xylene as a precursor compound, opening up numerous possibilities in chemical synthesis.
The ability to generate several valuable compounds from m-xylene gives it a prime role in chemical industries. The main advantage lies in the diverse array of derivatives that can be synthesized, offering various functional properties for different applications. However, potential difficulties related to process optimization and efficiency must be managed to improve overall yield in industrial settings.
In Fuel Formulations
m-Xylene's presence in fuel formulations helps to enhance the octane rating, thus improving engine performance. It serves as an effective blending component with other petroleum products, ensuring better combustion and fuel efficiency.
Using m-xylene in fuel can also lower exhaust emissions. However, there are safety considerations that need addressing, as m-xylene is harmful if inhaled or ingested in large quantities. Balancing its benefits with safety in handling and environmental regulations continues to be a crucial aspect in its application in fuel formulation.
Environmental Impact of m-Xylene
The impact of m-xylene on the environment is a critical area of consideration, particularly in discussions surrounding industrial chemicals. m-Xylene is widely used in various applications, raising concerns about its potential effects on ecosystems and human health. Understanding these impacts helps address risks related to its use and informs regulations aimed at mitigating negative effects.
It is essential to examine both direct and indirect influences of m-xylene on the environment. Its propensity to contaminate both air and water sources poses significant challenges to environmental safety and public health. Regulatory frameworks play a crucial role in controlling emissions and ensuring safe use in industrial processes.
Ecosystem Effects
Water Contamination
Water contamination from m-xylene primarily occurs through industrial discharge and improper disposal practices. When m-xylene enters water bodies, its presence can disrupt aquatic ecosystems. This hydrocarbon is toxic to aquatic life, affecting fish and other organisms. The key characteristic of water contamination by m-xylene is its persistence in the environment. Once introduced, m-xylene can remain in water for extended periods, continuously posing threats to living organisms.
Implications of water contamination:
- Impaired growth and reproduction of aquatic species.
- Potential for bioaccumulation in the food chain, leading to long-term ecological impacts.
- Health risks to humans who depend on contaminated water sources for drinking or recreation.
Thus, examining water contamination helps highlight the necessity for stringent monitoring and the implementation of effective waste management practices.
Air Pollution
Air pollution resulting from m-xylene emissions is another significant environmental concern. The compound can evaporate into the atmosphere, contributing to air quality degradation. The key characteristic of air pollution involving m-xylene is that it includes volatile organic compounds (VOCs). These VOCs can lead to the formation of ozone at ground level, a harmful pollutant with various health effects.
Effects of air pollution include:
- Respiratory issues, including asthma and other respiratory ailments.
- Reduced visibility and nuisance odors.
- Contribution to broader issues like climate change and urban smog.
Ultimately, both water and air contamination related to m-xylene require comprehensive strategies to reduce emissions and protect ecosystems.
Regulations and Guidelines

Regulatory frameworks are essential for controlling the environmental impact of m-xylene. National and international standards guide industries in managing m-xylene's use and related emissions. These regulations aim to minimize health risks and environmental degradation.
National Regulations
National regulations often dictate permissible limits for m-xylene in emissions and discharges. Agencies like the Environmental Protection Agency in the United States enforce strict guidelines on industrial practices. The key characteristic of national regulations is their localized enforcement, which allows countries to tailor approaches based on specific environmental conditions and public health needs.
Benefits of strong national regulations include:
- Creation of safe operational standards for industries.
- Reduced incidence of health problems linked to exposure.
- Encouragement of cleaner production technologies.
These regulations ensure that m-xylene's impact on the environment is continuously monitored and mitigated.
International Agreements
International agreements play a pivotal role in managing transboundary pollution issues related to m-xylene. Such agreements foster cooperation between nations to address the challenges posed by pollutants that do not respect political boundaries. An example is the Stockholm Convention, which addresses the elimination of persistent organic pollutants.
Advantages of engaging in international agreements:
- Standardization of policies and practices related to m-xylene management.
- Shared resources and knowledge among participating countries.
- A global commitment to reducing environmental and health risks associated with hazardous chemicals.
Health Considerations Related to m-Xylene
The evaluation of health considerations related to m-xylene is essential for understanding its safe handling and usage. m-Xylene is a widely-used solvent in various industrial applications, making awareness of its health risks paramount. Individuals working with m-xylene need to be informed of its toxicological effects and the necessary safety precautions to mitigate exposure. Recognizing these factors ensures that the benefits of m-xylene can be utilized without compromising worker safety or public health.
Toxicological Profile
m-Xylene is known for its potential health hazards, particularly when inhaled or absorbed through the skin. Its toxicity stems from various possible effects on the nervous system, kidneys, and liver. Short-term exposure can result in headaches, dizziness, and respiratory problems. Long-term exposure carries risks of more severe outcomes, including neurological damage and potential carcinogenic effects.
An assessment of m-xylene’s toxicity reveals its classification as a possible human carcinogen by organizations such as the International Agency for Research on Cancer (IARC). This underscores the importance of risk awareness among workers and regulatory bodies, ensuring that appropriate measures are taken to minimize exposure.
Safety Measures
Safety measures regarding m-xylene involve a comprehensive approach focusing on exposure limits and personal protective equipment. The aim is to establish a safe working environment within industries that utilize this compound.
Exposure Limits
Exposure limits for m-xylene are key guidelines established to protect workers in environments where exposure may occur. These limits dictate the maximum concentrations allowed in the air over a specific time, commonly expressed in parts per million (ppm). Typically, regulatory agencies, like the Occupational Safety and Health Administration (OSHA), have set permissible exposure limits (PEL) to safeguard health.
The importance of exposure limits lies in their role in preventing immediate and chronic health issues associated with inhalation of m-xylene fumes. They serve as a standard for ensuring that workplaces maintain air quality conducive to worker safety. Exposure limits may vary by region, depending on local regulations and practices. Thus, understanding these variances is crucial for both international operations and compliance.
Personal Protective Equipment
The use of personal protective equipment (PPE) is another integral safety measure. PPE consists of gear worn to minimize exposure to hazardous substances. For m-xylene, PPE recommendations generally include gloves, respirators, and protective clothing.
The key characteristic of PPE is its ability to provide a physical barrier between the worker and the harmful substance. Wearing appropriate PPE significantly reduces the risk of skin contact and inhalation, thereby enhancing overall safety in environments where m-xylene is used.
However, the effectiveness of PPE hinges on proper training and maintenance. Workers must be educated about the correct usage, selection, and limitations of the equipment they utilize. Improper usage can lead to a false sense of security, potentially elevating health risks.
In summary, understanding the health considerations related to m-xylene underscores its importance in industrial settings. By emphasizing the toxicological profile, adhering to exposure limits, and implementing robust safety measures, risks can be mitigated effectively. This balance facilitates not only the utilization of m-xylene's benefits but also the preservation of worker and environmental health.
Future Research Directions
The exploration of m-xylene is a continually evolving field. As industries shift towards sustainable practices and advanced applications, the necessity for dedicated research on m-xylene becomes apparent. Future research is essential not only for the better understanding of its properties and effects but also for the development of innovative applications and safer alternatives.
Innovative Uses of m-Xylene
Research into innovative uses of m-xylene can lead to breakthroughs in various fields. For instance, one area of focus could be the utilization of m-xylene in creating advanced materials with enhanced properties. These materials could revolutionize industries such as electronics, where lightweight and high-performance components are in demand. Moreover, m-xylene's role in the production of specialty chemicals could be expanded. Finding new catalysts for reactions involving m-xylene could improve efficiency and yield of important compounds.
Furthermore, studying m-xylene as a feedstock in biorefineries can open avenues towards greener chemistry. Integrating m-xylene in the synthesis of bio-based products could minimize reliance on fossil fuel-derived materials.
Sustainability and Alternatives
As the world increasingly scrutinizes the environmental impact of chemical substances, sustainability in the context of m-xylene is crucial. Future research should focus on finding sustainable alternatives to m-xylene derived from renewable resources. Developing bio-based pathways for m-xylene synthesis represents a significant step forward. Such alternatives could address both environmental concerns and market demand.
Additionally, research into the biodegradability of m-xylene and its byproducts is necessary. This knowledge could lead to improved practices in waste management and contamination remediation.
"Innovating the future requires a clear understanding of material impacts and sustainable practices."
In summary, the future of m-xylene research holds promise for innovations that align with environmental sustainability and the growing trend of responsible chemistry. It is only through addressing these questions that the chemistry and industry can evolve beyond current limitations.



